Background: Belantamab mafodotin (belamaf) is a monomethyl auristatin-F containing antibody drug conjugate that targets BMCA on myeloma cells. Single agent belamaf has shown clinical meaningful activity in relapsed/refractory multiple myeloma (RRMM) but ocular adverse events (AEs) affect 53-72% of trials'participants. In DREAMM-2, prophylactic corticosteroid eye drops did not effectively reduce the risk of keratopathy. It is therefore imperative that strategies to mitigate corneal AEs be explored.
This 2-part study is designed to evaluate belamaf and pomalidomide/dexamethasone (Pd) in RRMM. Here we report the efficacy and ocular AEs of patients (pts) receiving 2.5 mg/kg (maximum tolerated dose) or 1.92 mg/kg IV belamaf and administrated on various schedules aimed to define the optimal balance of efficacy and tolerability. We also present results of an ocular sub-study exploring the use of bandage contact lens (BCLs) to reduce the risk and severity of belamaf-associated ocular AEs.
Methods: Eligibility: > 1 prior lines of therapy (LoT), lenalidomide, proteasome inhibitors exposed, and refractory to the last LoT. Pd was administered per standard of care and belamaf (1.92 or 2.5 mg/kg) Q4W or 2.5 mg/kg Q8W, Q12W or SPLIT equally on days 1 and 8, Q4W. Pts enrolled on the ocular sub-study received the recommended part two dose (RP2D): 2.5 mg /kg Q8W. A BCL (Bausch and Lomb Pure Vision) was placed in the right (R) eye for the 1st 2 doses of belamaf with the left (L) eye serving as control. Subsequently, BCLs were placed in both eyes. Pts wore BCLs for up to 28 days after each belamaf dose. Evaluation by an eyecare specialist for a comprehensive eye exam and BCL placement occurred every 15-28 days during BCLs use and prior to each belamaf dose. Corneal findings were graded (Gr) by the keratopathy and visual acuity (KVA) scale and findings of local infection were reported. Keratitis prophylaxis during BCLs use included gatifloxacin 0.3% or tobramycin 1.3% eye drops.
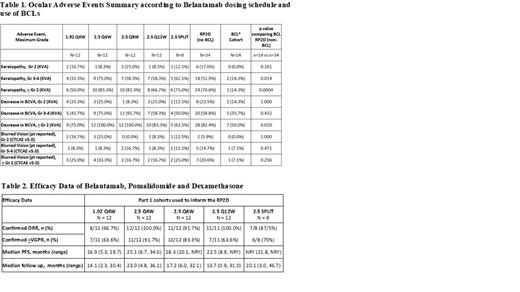
Results: This analysis comprises 92 pts including 14 on the ocular sub-study. Baseline best corrected visual acuity (BCVA) and ocular comorbidities were similar across all cohorts: with median 20/20 BCVA 46.7% R and 50% L eye. Abnormal fundoscopy was 21.7% in R and 16.3% in L eye.
There was a trend to lower incidence and less severity of ocular AEs for the 1.92 mg/kg dose (Table 1). Gr 3-4 keratopathy, decrease in BCVA and subjective Gr >2 blurred vision by CTCAE were 33.3% (4/12), 41.7% (5/12) and 25% (3/12) for the 1.92 mg/kg and 75% (9/12), 75% (9/12) and 33.3% (4/12) for 2.5 mg/kg Q4W cohorts. Preliminary data indicated superior clinical efficacy (ORR, >VGPR rate and mPFS) for the 2.5 mg/kg dose, 100% (12/12), 91.7% (11/12) and 25.1 months (11.8-NYR) versus 66.7% (8/12), 63.6% (7/12) and 16.9 months (5.3-19.7) for the 1.92 mg/kg dose (Table 2).
To preserve efficacy but reduce ocular AEs additional cohorts exploring 2.5 mg/kg dosed every 8 and 12 weeks were included. Extended dosing schedules showed lower rates of Gr 3-4 keratopathy, decrease in BCVA, and Gr ≥2 blurred vision: 58.3% (7/12), 91.7% (11/12) and 16.7% (2/12) of pts treated Q8W; and 58.3%, 58.3% and 16.7% (2/12) of pts treated Q12W versus the Q4W schedule. Importantly, the efficacy was preserved with ORRs of 91.7% (11/12) and 100% (11/11), >VGPR rates of 83.3% (10/12) and 63.6% (7/11) and mPFS of 18.3 months (95% CI: 10.1-NYR) and 22.5 (95% CI: 8.9-NYR) for Q8W and Q12W groups, respectively.
In the ocular sub-study, assessment of R and L eye during the first 2 belamaf doses showed a significant delay in onset of BCVA decrease in the R eye (p<0.0001) compared to the L. Comparing pts treated at the RP2D, moderate to severe (Gr ≥2) keratopathy and decrease BCVA were significantly lower (p=0.0004 and 0.010), in the BCL cohort versus no-BCL group. Further there was a trend towards less symptomatic blurred vision (p=0.256). BCL pts were able to complete 90% of the pre-planned belamaf cycles compared to 70% in the RP2D no-BCL group (p=0.029). No cases of infectious keratitis were reported in the BCL cohort.
Conclusions: Our data suggest that extended dosing intervals may be a significant step forward in mitigating corneal AEs of belamaf. This preliminary report on the efficacy of BCLs in 14 pts demonstrates a decrease in moderate to severe corneal AEs and reduction in dose holds for corneal AEs, noting however a short follow up at this time. The data support the further evaluation of BCLs in preventing belamaf associated ocular toxicities.
Disclosures
Louzada:Janssen: Honoraria; BMS: Honoraria; GSK: Research Funding; Forus: Honoraria. Reece:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Millenium: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. McCurdy:Amgen: Honoraria; GSK: Honoraria; Pfizer: Consultancy, Honoraria; Forus therapeutics: Consultancy, Honoraria; Sanofi: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Celgene: Honoraria. Kotb:Janssen: Honoraria; BMS: Honoraria; Amgen: Honoraria; Akcea: Honoraria; Merck: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Celgene: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; Karyopharm: Current equity holder in private company; Forus: Honoraria. Othman:Amgen: Honoraria; BMS / Celgene: Honoraria; Forus: Honoraria; Janssen: Honoraria; Pfizer: Honoraria; Sanofi-Genzyme: Honoraria; Takeda: Honoraria. Mian:Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GSK Awards: HHS Research Early Career Award from Hamilton Health Sciences Foundation: Honoraria; Celgene: Honoraria. Chu:Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Roche: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Amgen: Honoraria. White:Amgen: Consultancy, Honoraria; Antengene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Forus: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Takeda: Consultancy, Honoraria. Trudel:Sanofi: Honoraria; Genentech: Research Funding; Janssen: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; GSK: Consultancy, Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Roche: Consultancy, Research Funding; BMS: Consultancy, Honoraria, Research Funding; FORUS: Consultancy; K36: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal